

Company Profile
Jafron Biomedical Co., Ltd. (Stock Code: 300529), founded in 2002, is the first ChiNext-listed company specializing in blood purification therapy in China. Jafron leverages proprietary resin hemoadsorption technology to provide comprehensive solutions for chronic renal disease, liver failure, intoxication, and autoimmune disorders, with applications further extending to sub-health interventions.
Jafron has diligently devoted to the R&D, production, and provision of blood purification products primarily in the hemoadsorption field for decades. With more than 20 years of clinical experience, the company's flagship product, "HA series disposable hemoperfusion cartridges", originally developed by Jafron, has been adopted in more than 8,000 key hospitals across 99 countries and regions, with 6,000,000 cases of clinical application annually.
Jafron has set up around 130 domestic offices, with a professional therapy-providing team of over 1,400 staff and 1,000 partners. As a Chinese A-share listed company with a market value of $2['5'] billion, Jafron has 5 branches, 12 subsidiaries, 1 sub-subsidiary and also has operations covering other fields including medical foundation and insurance.
As a global adsorption therapies provider,Jafron will keep exploring novel medical fields to save lives, improve patients' quality of life, and realize the vision of "Health Technology for a Better World."

Main products
HA series hemoperfusion cartridge, BS bilirubin adsorption column and DNA immunoadsorption column, Hemoperfusion Machine and Blood Purification Machine, etc.
Company Profile

Jafron Biomedical Co., Ltd. (Stock Code: 300529), founded in 2002, is the first ChiNext-listed company specializing in blood purification therapy in China. Jafron leverages proprietary resin hemoadsorption technology to provide comprehensive solutions for chronic renal disease, liver failure, intoxication, and autoimmune disorders, with applications further extending to sub-health interventions.
Jafron has diligently devoted to the R&D, production, and provision of blood purification products primarily in the hemoadsorption field for decades. With more than 20 years of clinical experience, the company's flagship product, "HA series disposable hemoperfusion cartridges", originally developed by Jafron, has been adopted in more than 8,000 key hospitals across 99 countries and regions, with 6,000,000 cases of clinical application annually.
Jafron has set up around 130 domestic offices, with a professional therapy-providing team of over 1,400 staff and 1,000 partners. As a Chinese A-share listed company with a market value of $2['5'] billion, Jafron has 5 branches, 12 subsidiaries, 1 sub-subsidiary and also has operations covering other fields including medical foundation and insurance.
As a global adsorption therapies provider,Jafron will keep exploring novel medical fields to save lives, improve patients' quality of life, and realize the vision of "Health Technology for a Better World."
Main products
HA series hemoperfusion cartridge, BS bilirubin adsorption column and DNA immunoadsorption column, Hemoperfusion Machine and Blood Purification Machine, etc.

Dong Fan
Chairman of the Board
Dong Fan, currently chairman, president, and chief executive officer of Jafron, is responsible for its strategic direction, overall performance, and long-term growth plan. He is proficient in business management and marketing with excellent commercial awareness, a sense of technological innovation and market development, and a powerful consciousness and ability to combine social resources.
Mr. Dong graduated from the Shanghai University of Finance and Economics with a bachelor's degree in trade and economics in 1992, graduated from Sun Yet-Sen University with a master's degree in business management. Prior to steering Jafron in 2002, Mr. Dong was engaged in marketing and management in domestic famous pharmaceutical enterprises.
Dong Fan
Chairman of the Board

Dong Fan, currently chairman, president, and chief executive officer of Jafron, is responsible for its strategic direction, overall performance, and long-term growth plan. He is proficient in business management and marketing with excellent commercial awareness, a sense of technological innovation and market development, and a powerful consciousness and ability to combine social resources.
Mr. Dong graduated from the Shanghai University of Finance and Economics with a bachelor's degree in trade and economics in 1992, graduated from Sun Yet-Sen University with a master's degree in business management. Prior to steering Jafron in 2002, Mr. Dong was engaged in marketing and management in domestic famous pharmaceutical enterprises.
-

2021
Jafron established the MOST (Multi-organ Support Therapy) Global Advisory Board, which includes the world's top medical experts in clinical research and critical care medicine.
-
2020
Jafron hemoperfusion technology was included in 11 national medical manuals and recommendations for the treatment of Covid-19.
-

2018
The Multi-Center RCT Research of HA130 Hemoperfusion Cartridge was completed and obtained significant achievements.
-

2016
Jafron was listed on the A-share market.
Jafron launched DPMAS technology which was incorporated into the "Artificial Liver Treatment Guidelines." -
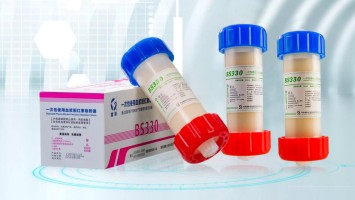
2012
BS Disposable Plasma Bilirubin Perfusion Adsorption Column for hepatopathy was launched.
-

2011
● Jafron successively owned CE Certification, ISO International Quality Certification, and China GMP National Certification.
● Jafron was listed in Forbes of Potential Chinese Enterprises and honored with "Key Hi-Tech Enterprise of National Torch Program."
-

2009
Jafron won the National Scientific and Technological Progress Award prize, the top-level technological award in China.
-

2007
Jafron Hemoperfusion Machine and Blood Pump were available to the public.
-

2005
DNA Immunoadsorption Column for the immune disease was launched.
-

2004
Jafron invented HA130 Disposable Hemoperfusion Cartridge for end-stage renal disease (ESRD) patients.
-

1989
Company was founded.
-

2021
Jafron established the MOST (Multi-organ Support Therapy) Global Advisory Board, which includes the world's top medical experts in clinical research and critical care medicine.
-

2020
Jafron hemoperfusion technology was included in 11 national medical manuals and recommendations for the treatment of Covid-19.
-

2018
The Multi-Center RCT Research of HA130 Hemoperfusion Cartridge was completed and obtained significant achievements.
-

2016
Jafron was listed on the A-share market.
Jafron launched DPMAS technology which was incorporated into the "Artificial Liver Treatment Guidelines." -

2012
BS Disposable Plasma Bilirubin Perfusion Adsorption Column for hepatopathy was launched.
-

2011
● Jafron successively owned CE Certification, ISO International Quality Certification, and China GMP National Certification.
● Jafron was listed in Forbes of Potential Chinese Enterprises and honored with "Key Hi-Tech Enterprise of National Torch Program."
-

2009
Jafron won the National Scientific and Technological Progress Award prize, the top-level technological award in China.
-

2007
Jafron Hemoperfusion Machine and Blood Pump were available to the public.
-

2005
DNA Immunoadsorption Column for the immune disease was launched.
-

2004
Jafron invented HA130 Disposable Hemoperfusion Cartridge for end-stage renal disease (ESRD) patients.
-

1989
Company was founded.

























































